Proteins
- Generalities (this section):
- Next sections:
|
Amino acids & Essential amino
acids |
|

|
Proteins are composed mostly of amino acids linked with peptide
bonds and cross linked between chains with sulphydral and hydrogen bonds.
Twenty different types of amino acids occur naturally in proteins. Proteins
differ from each other according to the type, number and sequence of amino
acids that make up the polypeptide backbone. As a result they have different
molecular structures, nutritional attributes and physiochemical properties.
Proteins are important constituents of foods for a number of different reasons.
They are a major source of energy, as well as containing essential amino-acids,
such as lysine, tryptophan, methionine, leucine, isoleucine and valine, which
are essential to human health, but which the body cannot synthesize.
Unlike
by plants, most amino acids cannot be synthesised by animals. Those that cannot
be synthesised are called essential amino acids (EAA) and have animals
have to rely on ingesting them through their diet (either from plants or from
other animals which already contain them) or on their synthesis by gut
bacteria. For fish and crustaceans the EAA's are arginine, histidine,
isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan
and valine.
|
Structure of amino acids |
|

|
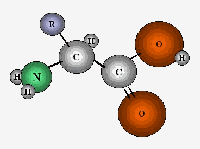
| An amino acid is a small organic
molecule that, as the name indicates, contains both an amino component and an
acid component. "Amino" refers to the group –NH2 (in green) in an organic
molecule, nitrogen bound to two hydrogens. The acid part of an amino acid is
the carboxyl group. |
| The middle carbon of the amino acid has an "R"
attached to it. In the case of amino acids, we refer to the R groups as "side
chains." There’s a virtually limitless variety of side chains that can be
used to construct an endless array of amino acids. But on earth we only find
about twenty amino acids in living systems. They all have the basic structure
but their side chains give each of them particular physical and chemical
properties. For instance, the aliphatic amino acids have side chains consisting
merely of carbon and hydrogen chains. These side chains are chemically inert,
and poorly soluble in water. In contrast, the acidic amino acids have side
chains with organic acids on them. These side chains are chemically reactive
and more water-soluble. |
 |
| Amino acid structure |
|
|
The 20 different amino acids |
|

|
| Non-polar
aliphatic R-groups |
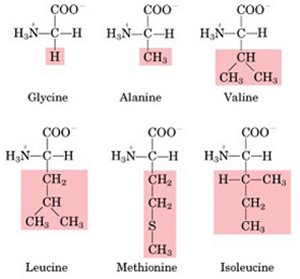 |
| Polar uncharged
R-groups |
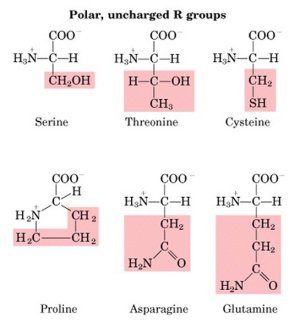 |
|
| Aromatic
R-groups |
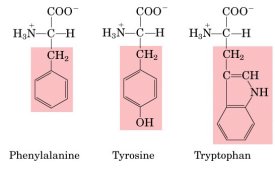 |
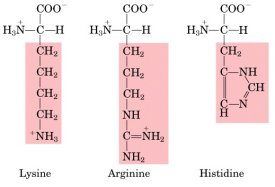
| Positively charged
(= basic) R-groups |
 |
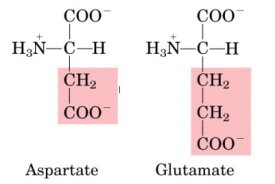
| Negatively charged
(= acidic) R-groups |
 |
|
|
Structure of proteins |
|

|
Proteins have four levels of structure, and looking at these
four levels is a useful and easy way to proceed.
- The first level of structure is called primary
structure. The primary structure of a peptide or protein is simply the sequence
of amino acids.The sequence of amino acids determines the structural and
functional characteristics of the protein. Proteins with very different
sequences of amino acids (different primary structures) will have very
different properties.
- Secondary structure refers to the spatial folding of
polypeptide chains. Depending on the sequence of amino acids, a polypeptide
chain can fold in a number of ways. This folding will be driven in part by the
tendency of hydrophobic side chains to minimize their contact with water and
hydrophilic side chains to maximize their contact with water. Hydrogen bonding
and the physical properties of the polypeptide backbone also play an important
role. Several patterns are very common, the most notable being alpha helices
and beta sheets. In alpha helices, the polypeptide backbone of the protein
coils into a helical configuration.
 |
| Four levels of the structure of proteins |
- Tertiary structure refers to the overall geometry of
the individual polypeptide, the way the secondary structure is itself folded.
Think of it this way: you have a straight length of telephone cord, marked off
into regular intervals, with each interval representing an amino acid. This is
the primary structure—the sequence of amino acids. Now imagine the phone
cord as it is allowed to naturally assume its coiled configuration. It’s
still stretched out to length, but its taken on a decidedly helical
configuration-- the secondary structure. Now, take that coiled phone cord and
roll it up into a compact wad. That’s the tertiary structure—the
overall geometry of the protein, which encompasses and is influenced by the two
levels of structure beneath it. It’s the primary structure of a protein
that determines how it will fold into secondary and tertiary structures, and
it’s the folding and overall shape of the protein that determines how that
protein will function.
- Quaternary structure refers to the way individual
polypeptides combine to form complexes. Many proteins actually are made up of
several polypeptides. The classic example is hemoglobin. Hemoglobin is a
tetramer, consisting of two alpha-globin proteins and two beta-globin proteins.
|






